Antibacterial, antifungal and in vitro cytotoxic activities of three extracts isolated from mint发表时间:2023-08-24 11:43
Full Length Research Paper Antibacterial, antifungal and in vitro cytotoxic activities of three extracts isolated from mint Dongbo Liu1,2,3,4, Liqin Hu1,2, Xuehui Liu1,2, Xincong Kang1,2, Yongquan Hu1,2, Hongqi Xie1,2, Zhilan Xia5 and Ling Xie1* 1Horticulture and Landscape College, Hunan Agricultural University, Changsha, P. R. China. 2State Key Laboratory of Subhealth Intervention Technology, Changsha, P. R. China. 3Hunan Provincial Key Laboratory of Crop Germplasm Innovation and Utilization, Hunan Agricultural University, Changsha, P. R. China. 4Hunan Co-Innovation Center for Utilization of Botanical Functional Ingredients, Changsha, P. R. China. 5Hunan Engineering Research Center of Edible Fungi, Changsha, P. R. China. Received 25 March, 2016; Accepted 3 August, 2016 A number of reports have been focusing on chemical compositions and functional properties of essential oils isolated from mint. However, there is little data available on the biological activities of non-volatile constituents. In this study, the antibacterial (against 2 gram-positive bacterial strains: Staphylococcus aureus and Bacillus cereus; 2 gram-negative bacterial strains: Escherichia coli and Pseudomonas aeruginosa), antifungal (against Candida albicans, Colletotrichum gloeosporioides and Penicillium polonicum) and cytotoxic (against Human Embryonic Kidney 293 cell line) activities of three non-volatile extracts (Extracts 1 to 3) from the leaves and stems of mint were evaluated. Extract 1 showed significant antibacterial activity against gram-positive pathogens S. aureus and B. cereus, with an inhibition zone of 12.60 and 12.08 mm, respectively. The minimal inhibitory concentration (MIC) of extract 1 against S. aureus was 0.94 mg/ml. On the other hand, it exhibited low cytotoxic activity against normal Human Embryonic Kidney 293 cell line (CI50 nearly 30 mg/ml), which suggested that Extract 1 could be a potential and safe antibacterial agent. Nevertheless, the tested gram-negative bacteria and pathogenic fungi were not susceptible towards Extracts 2 and 3. Key words: Mint, antibacterial activity, antifungal activity, cytotoxicity. INTRODUCTION Antibiotic resistance (ABR) has become a global threat to public health systems due to the misuse and abuse of antibiotics (Ferri et al., 2015). Development of novel antibiotics is one of the effective measurements to address this ABR challenge (Saleem et al., 2010). Medicinal plants are potential sources of antibiotics due to the immense varieties of functionally relevant secondary metabolites (Ngo et al., 2013; Saleem et al., 2010). Systematic screening of these natural products may result in discovery of novel effective antibiotics. Plants from the genus Mentha are usually aromatic, stimulant and carminative, and are extensively used in the pharmaceutical, food, beverages, cosmetics, and allied industries (Johnson et al., 2011). As a traditional medicine, it is often used for treating nerve center, breath and digestive diseases (Thompson and Ernst, 2002). This genus includes 25 to 30 species that grow in the temperate regions of Eurasia, Australia and South Africa, with at least 7 species endemic to Australia ((Lawrence et al. 2006; Tang et al., 2016). Considerable chemical diversities and biological activities are observed in the mint’s essential oil (Ahmed et al., 2015; Mimica-Dukic and Bozin, 2008; Petretto et al., 2014; Snoussi et al., 2015). Petretto has analyzed chemical constituents of essential oil from Mentha sueveolens species by gas chromatography-mass spectrometry (GC-MS). It shows that essential oil’s major compounds are oxygenated monoterpene compounds (82.5%) and have a strong antimicrobial activity against all yeast strains (Petretto et al., 2014). The study by Snoussi et al. (2015) on Mentha spicata essential oil on Vibrio species biofilm inhibition and eradication reinforces its applicable possibility in the pharmaceutical or food industry as a natural antibiotic and seafood preservative against Vibrio contamination. Antioxidant and antibacterial activity of essential oil of Mentha longifolia and Mentha pulegium enable it to be good for cardiovascular and throat health (Ahmed et al., 2015). While a number of reports have been focusing on chemical compositions and functional properties of essential oils isolated from mint, there is little data available on the biological activities of non-volatile constituents (such as flavonoids and polyphenolic compounds), which are reported to have broad-spectrum biological and antimicrobial activities and could be good substitutes for existing antibiotic drugs. In this study, the antibacterial, antifungal and cytotoxic activities of three non-volatile extracts from the leaves and stems of mint (the overground parts of Mentha australis R. Br.) were evaluated with the aim of finding out natural products as antibiotics. MATERIALS AND METHODS Extracts preparation Three extracts (Extracts 1 to 3) were isolated from leaves and stems of Australian native mint by incubation with 60% ethanol at 80°C for 1 h. Extracts 1 and 2 were purified by macroporous adsorption resin of AB-8 with 4 bed volume (BV) 20 and 30% ethanol, respectively. Finally, the yield of flavonoid extract was 22 mg/g of dried material. With the corresponding reference standards of rutin and rosmarinic acid, the content of the total flavonoids and polyphenol were determined by ultraviolet and visible spectrophotometry. The content of flavonoids in Extract 1 was 90.35% and that in Extract 2 was 79.65%. Extract 3 was purified with macroporous adsorption resin of HPD-400 and the content of polyphenol was 35.47%. Antibacterial activity Bacterial strains and culture medium Both gram-positive and gram-negative rods (Staphylococcus aureus ATCC 6538, Bacillus cereus ATCC 11778, Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853) were selected as microorganisms according to their pathogenic origin. The Beef-Protein medium was chosen for all bacteria culture. Fungi strains and culture medium Candida albicans ATCC 10231, Colletotrichum gloeosporioides and Penicillium polonicum has been chosen to evaluate antifungal activity. C. gloeosporioides and P. polonicum were isolated in our laboratory and identified by ITS sequence analysis. All fungi strains have been cultured in Sabouraud Dextrose Agar medium. Disc diffusion method The disc diffusion method was applied to determine the antimicrobial activity against four bacteria and three fungal pathogens. Bacteria were cultivated at 37°C for 24 h, fungi at 28°C for 5 days and these pathogens were adjusted to approximately 106 CFU/ml. Sterile paper discs (6 mm diameter) impregnated with tested extracts were diluted in sterile water for 12 h and then placed onto the surface of inoculated plates. Ampicillin (25 µg/ml) was used as positive control against S. aureus while streptomycin (25 µg/ml) against other three bacteria strains. Water-soluble amphotericin B (50 µg/ml) was used as positive control against all fungal strains. The plates were left for 15 min at room temperature and then incubated at 37°C for 24 h (for bacteria) or 28°C for 5 days (for fungi). Then the antibacterial activity was evaluated by measuring the inhibition zone around the discs with caliper. Each test was carried out in triplicate. Micro-Well dilution assay MIC values were determined by a micro-well dilution method. A high-throughput 96-well micro plate assay procedure was used according to the method of (Bala et al., 2011). The inocula of the bacteria and fungi were adjusted to approximately 106 CFU/ml. Within each sterile 96-well micro plate, the first two rows contained 200 µl medium as sterility check. Test samples were loaded in the next rows with respective concentrations of 30.00, 15.00, 7.50, 3.75, 1.88, 0.94, and 0.47 mg/ml, all comprising 100 µl culture and 100 µl extract. Streptomycin, ampicillin and water-soluble amphotericin B with corresponding concentration were used as positive controls. The same volume of microbiological culture and pure medium were used as negative controls. The test was replicated three times in each run of the experiments. The plates were covered with sterile plate seal and then incubated for 24 h/48 h. The MIC was defined as the lowest concentration at which the extract could be able to inhibit any visible bacterial or fungal growth. Determination of MBC (MFC) From the determination of MBC, 0.1 ml liquid medium without bacterial growth had been gotten and coated uniformly onto the surface of inoculated plates. And then they were incubated for more than 24 h at 37°C/28°C and checked whether microorganism grew or not. The minimum mass concentration without microorganism growth was MBC value for bacteria and the minimal fungicidal concentration (MFC) for fungi. Cytotoxic activity Cell culture The normal Human Embryonic Kidney 293 cell line (293FT) were Table 1. The inhibition zone diameter (mm) of isolated extracts of different concentrations against tested bacteria.
NI, No inhibition observed; (+), gram-positive; (-), gram-negative; a, positive control; b, blank control. kindly provided by PhD Yanyang Wu from Tsinghua University and seeded at the density of 2×105 cells/60 mm culture dish (BD Discovery Labware, Bedford, Massachusetts) (Jain et al., 2011). Cells were incubated at 37°C in the culture medium of DMEM basic (Gibco Cat. C11995500BT) +10% Foetal Bovine Serum (BI Cat. 04- 001-1ACS) as culture medium in a humidified atmosphere of 95% air and 5% CO2. At the onset of the experiments, the cells were at an exponential and asynchronous phase of growth. Evaluation of cytotoxicity Cytotoxicity was evaluated with the cell counting kit-8 (CCK8, Dojindo) method. 100 µl cell suspensions were inoculated in the 96- well culture plate and incubated at 37°C. After treatment with various concentrations of extracts, cells were re-incubated for an additional 48 h at 37°C. Then the medium was discarded and the cells re-incubated for 2 h after 10 µl of CCK8 solution added. The CCK8 solution was then removed and 200 µl insoluble formazan crystals were added. The optical density (OD) was measured at 540 nm by using an enzyme-linked immunosorbent assay plate reader. Data were obtained from triplicate wells. The cytotoxicity index (CI%) was calculated according to the following equation: CI% = (C-T)/C×100, where T and C respectively represent the mean optical density of the treated group and vehicle control group (Selim et al., 2015). CI50 was defined as the concentration (µg/ml) of the substrate that causes 50% death of cells. HPLC analyses of flavonoids compounds The dry samples (100 μg) were redissolved in 100 µl of methanol and were later injected into an Agilent 1260 infinity series HPLC with an Agilent Zorbax SB-C18 column (250×4.6 mm, 5 µm) using acetonitrile and 0.5% acetic acid as the mobile phase (flow rate, 1 ml/min), and the detector was set at 254 nm. Chromatographic peaks were identified by comparing the retention times and spectra against the standards of diosmin (97%, Sigma). Statistical analysis Statistical analysis was performed by using analysis of variance (ANOVA). The Statistical Package for Social Sciences (SPSS) 18.0 software package (SPSS Inc., Chicago) was used to perform statistical analysis and P value of ≦0.01 was considered to be significant. RESULTS AND DISCUSSION Antibacterial activity The antibacterial activities of three non-volatile extracts with different concentrations were evaluated by using disc diffusion method against gram-positive (S. aureus ATCC 6538 and B. cereus ATCC 11778) and gram- negative (E. coli ATCC 25922 and P. aeruginosa ATCC 27853) bacterial strains. Two standard antibiotics (streptomycin and ampicillin) were used as positive controls in this assay. The antibacterial activity, which presented as a clear inhibition zone against these four bacterial species, is summarized in Table 1. At the same time, we have tested minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC) to evaluate the antibacterial activities of three extracts as shown in Table 2. Extract 1 was the most active one against bacteria among these three extracts (Tables 1 and 2). Especially, it showed significant antibacterial activities against gram- positive bacteria S. aureus and B. cereus, with inhibition zone of 12.60 and 12.08 mm and MIC of 0.94 and 7.50 mg/ml, respectively. Its inhibition effect was closely related with the dose (Table 1). The decreasing growth of Table 2. MIC and MBC for antibacterial activities of three extracts.
Note: MIC, minimum inhibitory concentration (mg/mL); MBC, minimum bactericidal concentration (mg/mL); -, not been tested. Table 3. The inhibition zone diameter (mm) of isolated extracts of different concentrations against tested fungi.
NI, No inhibition observed. the bacteria was observed with the increasing dose of compounds used. The highest antibacterial activity was observed against S. aureus by 30 mg/ml Extract 1, with an inhibition zone of 12.60 mm. Compared with the activities against gram-positive bacteria, the results of inhibition zone and MIC and MBC all showed that it had lower activity against gram- negative bacteria, even by Extract 1 (Tables 1 and 2). This finding agreed with previous reports describing the effectiveness of antimicrobial agents. This may be attributed to the porous nature of the outer peptide-glycan layer of cell wall of gram-positive bacteria that extracts could pass through the cell wall easier (Nostro et al., 2000). The concentration of extracts with no bacteria growing on the agar disc after 48 h incubation was considered as MBC. Except those against S. aureus, the MIC and MBC of three extracts against bacteria were at the same concentration. It suggested that the inhibited effects of these three compounds are long lived. With the prolonged incubation time, S. aureus was only susceptible at higher concentration. That maybe suggests that S. aureus could overcome the antibacterial effect of these three extracts. Antifungal activity The activities of three extracts against fungi (C. albicans, C. gloeosporioides and P. polonicum) are shown in Tables 3 and 4. The results of inhibition zone did not follow the pattern observed in the MIC or MFC Table 4. MIC and MFC for antifungal activities of three extracts.
MIC, Minimum inhibitory concentration (mg/mL); MFC, minimal fungicidal concentration (mg/mL); -, not been tested.
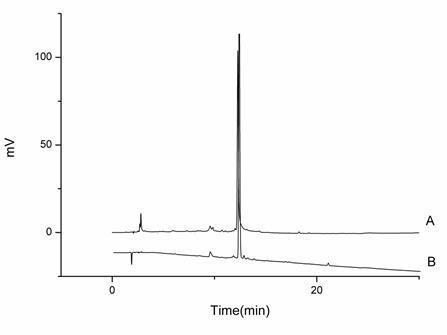
Figure 1. HPLC analysis of Extract 1. A, the standard of diosmin; B, Extract 1. determination. The biggest inhibition zone was shown as 8.86 mm by 30 mg/ml Extract 2 against C. albicans, while in the MIC and MFC results, Extract 1 showed more active, with the MIC/MFC at 7.5 and 15.0 mg/ml against C. gloeosporioides and P. polonicum, respectively. That may be ascribed to (1) the spores of C. gloeosporioides and P. polonicum were easy to auto-infect when cultured on the solid medium; (2) the extracts could be thoroughly exposed in the liquid medium. Consequently, the inhibited effect against C. gloeosporioides and P. polonicum could not be accurately measured on the agar discs. Though we have purified and obtained the diosmin, which was reported to inhibit the growth of Bacillus subtilis and the human pathogenic fungus Trichophyton rubrum, from Extracts 1 and 2 by HSCCC, and verified by HPLC with the reference standard (Figure 1), the antimicrobial activities of the extracts are difficult to correlate with diosmin due to their complexities and variabilities. Some reports considered that there may be
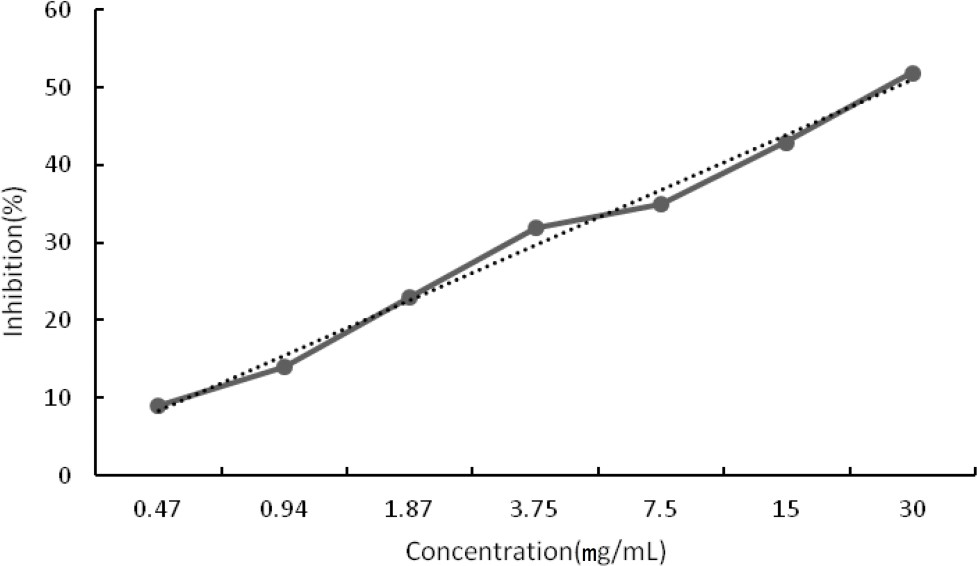
Figure 2. Cytotoxic activity of different concentrations of Extract 1 on Human Embryonic Kidney 293 cell line (HEK-293FT). some relation between the most abundant components of compounds and the antimicrobial activity (Oliveira et al., 2014). In this study, the content of flavonoids in Extract 1 was the highest (90.35%) in these three extracts. That may be why the antimicrobial effect of Extract 1 was the most significant. CCK8 cytotoxic activity A preliminary in vitro cytotoxicity assay against a normal cell line (Human Embryonic Kidney 293 cell line (HEK- 293FT)) was performed by using the Cell Counting Kit-8 (CCK8). The cytotoxicity of Extract 1 was determined at various concentrations, and expressed in half maximal cytotoxicity index (CI50, mg/ml) (Figure 2). The results showed Extract 1 exhibited low cytotoxic activity against human normal cell line with CI50 values approximately 30 mg/ml. CONCLUSION This study is the first report on the antibacterial, antifungal and cytotoxic activities of three non-volatile extracts from the leaves and stems of mint. Extract 1 showed antibacterial activity against various pathogens with the most significant activity against S. aureus. It exhibited low cytotoxicity against cell line 293FT with the CI50 approximately 30 mg/ml. Therefore, Extract 1 could be suggested as a potential and safe antibacterial agent. However, further research is necessary to establish the pharmacological mechanism of tested extracts. ACKNOWLEDGEMENTS This work was supported by the projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2012BAD33B11) and Program of International Science & Technology Cooperation of Ministry of Science and Technology (2013DFA31790). We would like to thank Mr. Zhou Ribao (Hunan University of Chinese Medicine, China) for identifying botanical and Dr. Yanyang Wu for providing cell as a gift. Conflict of interests The authors have not declared any conflict of interests. REFERENCES Ahmed AM, Ozbak, HA, Hemeg, HA (2015). Effect of essential oil of traditional two Saudi mint types and its possible role in cardiovascular and throat health. Int. J. Clin. Exp. Med 8:8060-8068. Bala N, Aitken EAB, Fechner N, Cusack A, Steadman KJ (2011). Evaluation of antibacterial activity of Australian basidiomycetous macrofungi using a high-throughput 96-well plate assay. Pharm. Biol. 49:492-500. Ferri M, Ranucci E, Romagnoli P, Giaccone V (2015). Antimicrobial Resistance: A Global Emerging Threat to Public Health Systems. Crit. Rev. Food Sci. Nutr. In press. Jain D, Pathak N, Khan S, Raghuram GV, Bhargava A, Samarth R, Mishra PK (2011). Evaluation of Cytotoxicity and Anticarcinogenic Potential of Mentha Leaf Extracts. Int. J. Toxicol. 30(2):225-36. Johnson M, Wesely EG, Kavitha MS, Uma V (2011). Antibacterial activity of leaves and inter-nodal callus extracts of Mentha arvensis L. Asian. Pac. J. Trop. Med 4:196-200. Lawrence BM (2006). The genus Mentha FL: CRC Press, Boca Raton 333 p. Mimica-Dukic N, Bozin B (2008). Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr. Pharm. Des. 14:3141-3150. Ngo LT, Okogun JI, Folk WR (2013). 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 30:584-592. Nostro A, Germano MP, D'Angelo V, Marino A, Cannatelli MA (2000). Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol. 30:379-384. Oliveira SD, Relison Tintino S, Gomes Figuer Edo F, Melo Borges MC, Bezerra Morais Braga MF, Bezerra Felipe CF, Martins Da Costa JG, Melo Coutinho HD, Alencar De Menezes IR, Regina Kerntopf M (2014). Atividade antibacteriana e moduladora de Cecropia pachystachya Trécul sobre a ação de aminoglicosídeos. Rev. Cub. Plantas Med. 19:121-132. Petretto GL, Fancello F, Zara S, Foddai M, Mangia NP, Sanna ML, Omer EA, Menghini L, Chessa M, Pintore G (2014). Antimicrobial activity against beneficial microorganisms and chemical composition of essential oil of Mentha suaveolens ssp. insularis grown in Sardinia. J. Food. Sci. 79:M369-M377. Saleem M, Nazir M, Ali MS, Hussain H, Lee YS, Riaz N, Jabbar A (2010). Antimicrobial natural products: an update on future antibiotic drug candidates. Nat. Prod. Rep. 27:238-254. Selim S, Amin A, Hassan S, Hagazey M (2015). Antibacterial, cytotoxicity and anticoagulant activities from Hypnea esperi and Caulerpa prolifera marine algae. Pak. J. Pharm. Sci. 28:525-30. Snoussi M, Noumi E, Trabelsi N, Flamini G, Papetti A, De Feo V (2015). Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio spp. Strains. Molecules 20:14402-14424. Tang KS, Konczak I, Zhao J (2016). Identification and quantification of phenolics in Australian native mint (Mentha australis R. Br.). Food Chem. 192:698-705. Thompson CJ, Ernst E (2002). Systematic review: herbal medicinal products for non-ulcer dyspepsia. Aliment. Pharmacol. Ther. 16:1689-1699. |